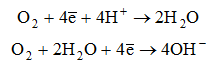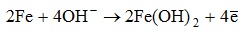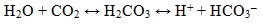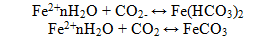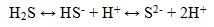Oil & Gas corrosion Lexicon
Corrosion of metal equipment in the oil and gas industry
Oilfield equipment corrosion occurs under the influence of mineral salts and corrosive aggressive gases such as hydrogen sulfide, carbon dioxide, hydrogen chloride and oxygen, which are dissolved in the formation and bottom water. Corrosion stimulating also possible by some chemicals fed into the well and bacteria (for example, sulfate-reducing bacteria), that may be contaminated bottom water with. This type of corrosion refers to the local corrosion and its different types are observed on the interior (the working surfaces of equipment). Also, the above mentioned equipment is exposed by total surface (solid) corrosion. It takes place on the outside of the equipment and flows under the influence of natural factors (moisture, oxygen, temperature, air emissions, etc.).
Total surface corrosion
The photos are examples of the general (solid) atmospheric corrosion of the production tree of oil and gas equipment.
As you can see at the photo, in this case corrosive destruction are distributed evenly across the surface of the metal. It is called total surface corrosion or solid corrosion. Basically, it occurs under the influence of atmospheric moisture (fog, rain, snow) and oxygen.
Corrosion occurs in the presence of oxygen according to the following mechanism:
a) cathodic process:
b) anodic process:
The solid corrosion is, of course, dangerous to metal equipment, but not so much as a local because its effects can be relatively easily removed and taken into account.
The most dangerous (local) types of corrosion of oil and gas equipment arise mainly under the influence of corrosive gases such as hydrogen sulfide (H2S) and carbon dioxide (CO2) which dissolved in the formation water and bottom water. Corrosion stimulating also possible by some chemicals fed into the well and bacteria (for example, sulfate-reducing bacteria), which are in the bottom water.
Corrosion of steel in environments containing carbon dioxide (CO2)
Dissolved in water, CO2 forms carbonic acid dissociates according to the reaction:
The high corrosion rate in carbon dioxide solutions explained by the fact that along with the H+ ions and HCO3 - a large number of undissociated H2CO3 molecules are in the solution which are buffer. The corrosion process involves not only the protons produced in the dissociation of H2CO3, but also the carbonic acid. The predominance of one or another particle serving as a depolarizer, is dependent primarily on the pH and medium composition. Iron bicarbonates and iron carbonate can form in CO2 containing solutions:
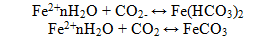
These products are easily eroded packless rust that does not create the protective film. That is, they practically do not slow down the process of corrosion, and rust formation is occurred directly at the surface of the metal. Carbonate deposits, formed on the steel surface, very packless and slightly prevents access corrosion agents, such as O2 and CO2, to the metal surface, which does lead to increase in the corrosion rate.
Incomplete coverage of the surface of the metal mentioned deposits (packless and porosity of the layer) stipulates the form of corrosion of metals.
The predominant form is a local corrosion and all of its features: pitch, pitting, corrosion cracking, intergranular, knife, etc.
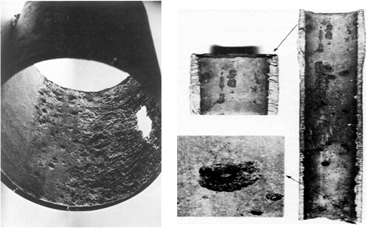
The photo shows examples of corrosion occurring in the gas equipment under the influence of CO2. This is carbon dioxide corrosion of pipes without the use of corrosion inhibitors. Typically, direct metal loss in this case is small, but on the internal surfaces, particularly shells, grooves, sores, fistula are formed in the lower part of the equipment and pipelines. This usually leads to considerable material and environmental losses associated with the cost of repairs, elimination of consequences of accidents, land reclamation, etc.
Corrosion of steel in containing hydrogen sulfide (H2S) environments
Hydrogen sulfide (H2S) , when its present in the crude oil and gas production wells, is very corrosive. It causes corrosion damage to equipment as a result of the electrochemical processes in the system and as a result of hydrogen embrittlement of the metal.
Hydrogen sulfide is an acid gas and partially dissociates to form hydrogen ions in the presence of water:
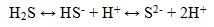
H2S is typically in the molecular form in the produced fluids from wells (bottom water), where the pH value below 7. However, if the H+ ions expend itself in the corrosion reaction, the forward and reverse reactions, catalyzed by various intermediates are very fast and iron corrosion rate are increasing.
The corrosion products formed in containing H2S environments on the corrosion rate can affect not equal. So pyrite (FeS2) and troilite (of FeS) create film on the metal, which has a some kind of corrosion-resistant (protective) action, and kansite (Fe9S8) has an imperfect crystal lattice. It does not prevent corrosion and does not have protective properties. As a result, in the presence of H2S pretty high corrosion rate is arisen in system.
Examples of corrosion occurring at the oil and gas field equipment under the influence of H2S
The photo shows examples of corrosion occurring at the oil and gas field equipment under the influence of H2S. The final result of this is the rapid destruction of the equipment due to the formation of pitting, ulcers, through holes, and the hydrogen absorption and metal embrittlement by diffusion of hydrogen atoms (H) into its crystalline lattice. Hydrogen embrittlement under static loading of metal (eg, tubing, intra- and inter-field pipelines) reduces its strength.
This phenomenon is called static hydrogen fatigue, and in H2S environments - it is sulfide stress cracking (SSC). It is experimentally proved that when the concentration of H2S in the system was from 25 mg/dm³ to 100 mg/dm³, the "lifetime" metal samples varied from 20 to 3.5 minutes. There are also a number of other factors that determined by the local types of corrosion, for example, galvanic corrosion, mill scale, stress concentration and local elements, the poor quality of the metal, mechanical damage to the surface of a metal of any type, the presence of bacteria in the formation water, etc.
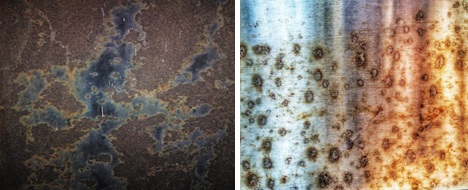
Bacterial corrosion
Among of many types of bacteria the greatest harm is generated by sulfur-reducing bacteria (SRB). Approximately 80% of corrosive wells damages is associated with SRB. The process of microbial corrosion occurs in the areas of equipment, where stagnant water (the bottom of the tank, casing, the inner surface of water mains, etc.). SRB throughout its life turns SO2 ³- and SO3 ²- ions into H2S, oxidizing molecular hydrogen (H2), presented in natural water or emitted in the cathodic reaction in the corrosion processes of steel equipment. If corrosion rate in saline deaerated water at normal temperature without H2S is 0.02 - 0.002 mm/year, in the case of presence only H2S in medium, corrosion rate reaches up to 0.3 - 0.5 mm/year. The actual corrosion rates of functional oil and gas producing equipment amount to 1.0 - 1.5 mm / year or above, because formation and effluent water contains not only H2S, but oxygen (O2). In some cases, there are the corrosion rates of 6 - 8 mm/year.
Bacterial corrosion under the SRB influence
Other important factors that affect the rate of corrosion of oil and gas equipment
In addition to the above, there are many other factors that have a profound impact on the nature and rate of corrosion. For example, the intensity of corrosion of steel decreases with the fall of acidity of the solution. When the solution becomes slightly alkaline, corrosion is reduced and there comes a natural inhibition. However, when the pH increases further, it is occurred phenomenon known as alkaline cracking, which can lead to disastrous results.
Increasing the salt content in the water is also typically accompanied by an increase in corrosion rate. An illustrative example of this is significant increase in permeability of sulfides with hydrogen sulfide (and hence the increasing of corrosion rate of ferrous metals) directly in salt solutions.
Significant impact on the corrosion process has a partial pressure of corrosive gas. For example, the corrosion rate with increasing of the hydrocarbon partial pressure of about 0.3 MPa quickly increases. There are evidences that with increasing in partial pressure of CO2 about 1.2 MPa, the corrosion rate can be up to 5.7 mm/year.
The corrosion rate increases rapidly with increasing temperature. This also increases the diffusion of oxygen and solution conductivity. Each of these factors results in a more rapid course of the corrosion process. Furthermore, probability of local damages of the metal in overheating spots increases with the formation scum or sludge on the heat transfer surface.
Stream velocity (Fluid Dynamics) is also important. Stationary state of the solution is not advantageous because it can lead to the formation of local corrosion damage. Increasing the flow rate usually increases the corrosion rate. Thus, the increase in gas-liquid flow rate of 2 to 8-10 m/s the rate of corrosion increases, and is then slowed down somewhat at a flow rate of 10-12 m/s the corrosion rate dramatically increases because of corrosion-erosion action factor. With decreasing partial pressure, the amount of corrosion and erosion region move toward a higher flow rate.
In conclusion, it should be emphasized that the main damage from the corrosion of metals is associated not only with the loss of large quantities of metal, but also from damage or complete failure of the metal processing equipment and structures, because of the corrosion they lose the necessary strength, flexibility, integrity, and thermal conductivity, and other necessary characteristics. It should be associated with the losses caused by corrosion, the following also: the enormous costs of any kind of protective anti-corrosion measures, the damage caused by the deterioration of the quality of produced products, reduced productivity of the equipment, accidents at the facilities, as well as economic and environmental losses due to measures for land reclamation and protection of human health and the environment.
Thus, anti-corrosion of oilfield equipment is the actual scientific and technical problem.